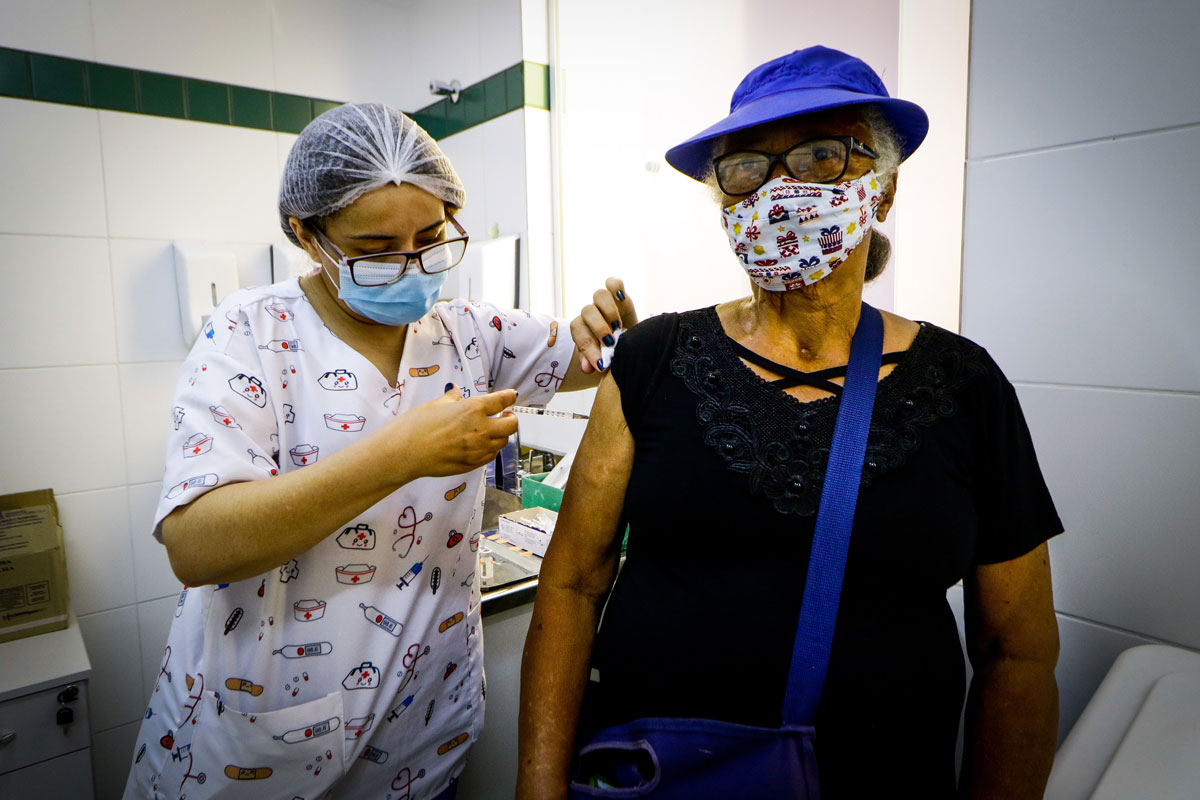
More good news on the vaccine front: the Food and Drug Administration just authorized the booster shot for people over 65 and at-risk. There have been many articles and reports released about the various vaccine’s efficacy rates at so many months out, which has led to speculation over the need for booster shots. It is now believed, with the Delta variant becoming dominant and other variants being watched, that booster shots will be needed for almost everyone. Originally, we were told boosters should be administered eight months after we became vaccinated. In August, Pres. Biden said they would most likely be able to offer boosters sooner. However, once those boosters became available, the FDA issued a statement recommending that the general population should not receive a booster at this time. On Wednesday, however, the booster was authorized for the elderly and high-risk population who received their 2nd dose at least six months ago.
The Food and Drug Administration has authorized a booster shot for Pfizer’s COVID-19 vaccine among some populations.
The single booster shot must be administered at least six months after completion of the first two doses. Those eligible to receive the booster include individuals 65 years of age and older, anyone older than 18 who is at high risk of severe COVID-19, and those 18 and over who are at risk of serious complications from COVID-19 due to high exposure at their job.
Some of the populations who fall under these categories are “health care workers, teachers and day care staff, grocery workers and those in homeless shelters or prisons,” acting FDA Commissioner Janet Woodcock, M.D., said in a statement Wednesday.
The latest authorization amends the FDA’s previous emergency use authorization for the Pfizer vaccine. In August, the initial two-dose series of Pfizer’s shot was granted full approval by the FDA for people 16 years and older.
Some of the populations who fall under these categories are “health care workers, teachers and day care staff, grocery workers and those in homeless shelters or prisons,” acting FDA Commissioner Janet Woodcock, M.D., said in a statement Wednesday.
The latest authorization amends the FDA’s previous emergency use authorization for the Pfizer vaccine. In August, the initial two-dose series of Pfizer’s shot was granted full approval by the FDA for people 16 years and older.
“This pandemic is dynamic and evolving, with new data about vaccine safety and effectiveness becoming available every day,” Woodstock said. “As we learn more about the safety and effectiveness of COVID-19 vaccines, including the use of a booster dose, we will continue to evaluate the rapidly changing science and keep the public informed.”
As predicted, the booster roll-out will likely mirror the initial roll out. It will go to the highest risk first and other age groups after those studies are completed. I included the last paragraph in the excerpt because again, the onslaught of information about vaccines is important. This virus, while it feels interminable, is new in terms of being studied as a disease and vaccine. And the constant monitoring and adjusting is vital for our safety. The back and forth, despite how Fox News and anti-vaxxers want to spin it, proves our very best minds are on top of it.
The bad news is I don’t have a definitive answer for you about mixing boosters. The booster authorized was the Pfizer booster. I can’t find if those who received the the Moderna vaccine can take the Pfizer booster. Moderna also has a booster submitted for authorization, that’s still pending. Johnson & Johnson recipients, however, may not have the Pfizer or Moderna boosters at this time. However, as CB reported, the J&J efficacy rate is still very high at this point. Obviously your medical professionals will know the correct information for you so check with them if you qualify for the booster. We can still hope for a semi-safe holiday season, y’all.
photos credit: Avalon.red












I was just discussing this last night with my parents. My father was vaccinated with Moderna and the doctor advised him to hold off on a Pfizer booster. Which I think is sound advice – for now.
From what I’m reading, it appears Moderna maintains its full effectiveness as time passes (well, at least through 120 days) better than Pfizer does, hence why boosters are being recommended after 6 months for people who’ve received Pfizer. There was a report about it released by the CDC last week but am having trouble with the link. My dad also received Moderna, and his doctor also said to hold off – that it remains to be seen (at least as of two weeks ago) if they will recommend boosters for people who received Moderna.
I got my third booster a couple weeks ago, right when the provisional recommendation came out and my doctors said definitively NOT to mix shots. So I made sure my CVS had Moderna before I went. You can look it up on the state and individual company websites, which shots are where.
Yes, the mixing of the shots is where the issue came up. I made sure when we went (for our second) to ask which one was being given.
I am super excited about this because both my husband and I qualify since I am in healthcare and he is a teacher. We are getting ours tomorrow morning and I am so relieved. I hope they approve it for everyone really soon.
I’m a teacher at a university that does not have a vaccine mandate. I’m hoping “teacher” includes universities.
I would say you qualify. I’m high risk and when I originally got vaxxed no one asked questions and it wasn’t available for everyone at that time.
In round one when supply was more of a concern, my friend who’s a PhD candidate and a TA qualified as a teacher; I know I want y’all as protected as possible!
I’m fat and have asthma so I’m high risk. I’ll need a booster at the end of next month.
I got my Pfizer booster over the weekend (high risk). This time the pharmacy did ask me to write down my condition and the pharmacist had to “approve” it. Other than that, it was easy-peasy. I had no side effects from shot 1&2 other than a sore arm. Booster gave me a much more sore arm but that went away in under 48 hours. I had a slight headache for a few hours and that was it.
In calif (Santa Barbara) I made an appt on CVS website and we got our Moderna boosters a few hours later. They had both vaccines available and main and only criteria for shot seemed to be if you were over 65 and Medicare. We were 7 months out from our second shot.
Good, I have to see about getting an appt for my mom (she’s in her mid 80’s).
Just tried to make an appt for her, but it’s still set up for only for adults with compressed immune systems. They haven’t updated it yet.
I got my third Pfizer shot last Sunday and got the flu and pneumonia vaxes as well.
My husband, physician that works with mostly elderly, walked into CVS and had the booster, but he played it as it was his first vaccination. He was vaccinated in December and he wasn’t waiting, as he is constantly having his patients about 6” from his face, retina specialist. He wasn’t taking any chances, as our patients here in Texas have been utterly disgusting “humans” in regards to the entire coronavirus restrictions and the advancement of the Delta variant. They refuse to follow practice restrictions regarding their positive status and are basically ugly and defiant with restrictions in place. They ALL act like it’s “no big deal” as to the jeopardy that they place on my husband and the staff. 🤬
My local Walgreens was offering boosters as soon as it started coming out that they’d be necessary. I went in that same day and got the Moderna booster. I have MS and don’t feel like risking it. They probably had surplus doses to shift or something.
I got my booster yesterday, CVS didn’t ask me a thing, just gave me the shot.